The arrangement of the experiment to show that electrons in motion exhibit wave-like properties is shown in figure. The apparatus consists of electron gun G where the electrons are produced and obtained in afine pencil of electron beam of known velocity. The electron gun consists of a tungsten filament F heated to dull red so that electrons are emitted due to thermionic emission. Now the electrons are accelerated in the electric field of known potential difference. After this the electrons are collimated by suitable slits to obtain a fine beam. The beam of electrons is directed to fall on a large single nickel crystal, known as target T. The electrons, acting like wave, are diffracted in different directions. The angular distribution is measured by an electron detector (Faraday cylinder

|
C) which is connected to a galvanometer. The Faraday cylinder can move on a circular graduated scale S between 200 to 900 to receive the reflected electrons. The Faraday cylinder consists of two walls which are insulated from each other. A retarding potential is maintained between them so that only fast moving electrons coming from electron gun may enter inside it. The secondary electrons (slow electrons) produced by collision with atoms from nickel target are reflected by Faraday cylinder. In this way the
|
galvanometer deflection is only due to electrons coming from electron gun.
The Faraday cylinder was moved on the circular scale and for a given accelarating voltage V,
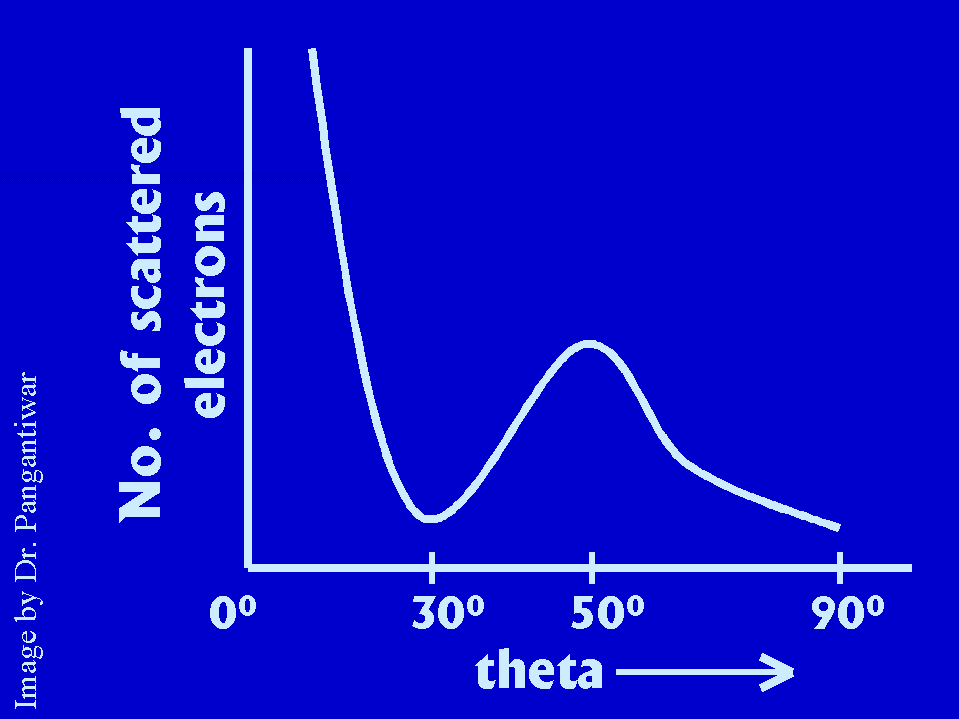
|
the scattering curve showed a peak in a particular direction theta. With the electron beam incident perpendicular to the crystal surface, the pronounced scattering direction was found to be 500 for electrons accelerated to 54 volts. Under these conditions, the surface row of atoms act like the ruling of a diffraction grating, producing the first order spectrum of 54 volts electrons at theta = 500.
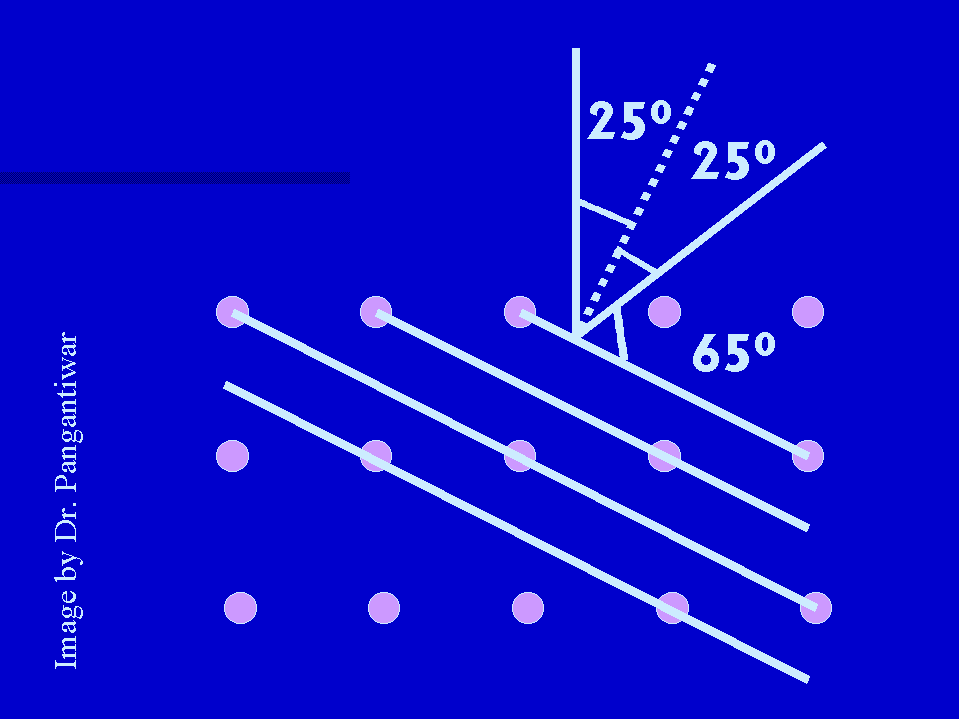
Since interatomic distance for nickel crystal is known to be 2.15 x 10-15 m, the interplaner distance d = 2.15 x 10-15 sin 250 = 0.09 x 10-10 m.
Using Bragg formula 2 x 0.909 x 10-10 sin(900 - 250) = 1 lamda.
Here angle theta is the angle between the incident beam and the interatomic plane.
So, lamda = 1.65 x 10-10 m = 1.65 A0
According to de-Broglie electron wave
lamda = 12.26 / (square root of 54) = 1.67 A0
As the two values are in good agreement, it is
|
confirmed that electrons in motion exhibit wave-like properties..
==============
By :- Dr. A. W. Pangantiwar


