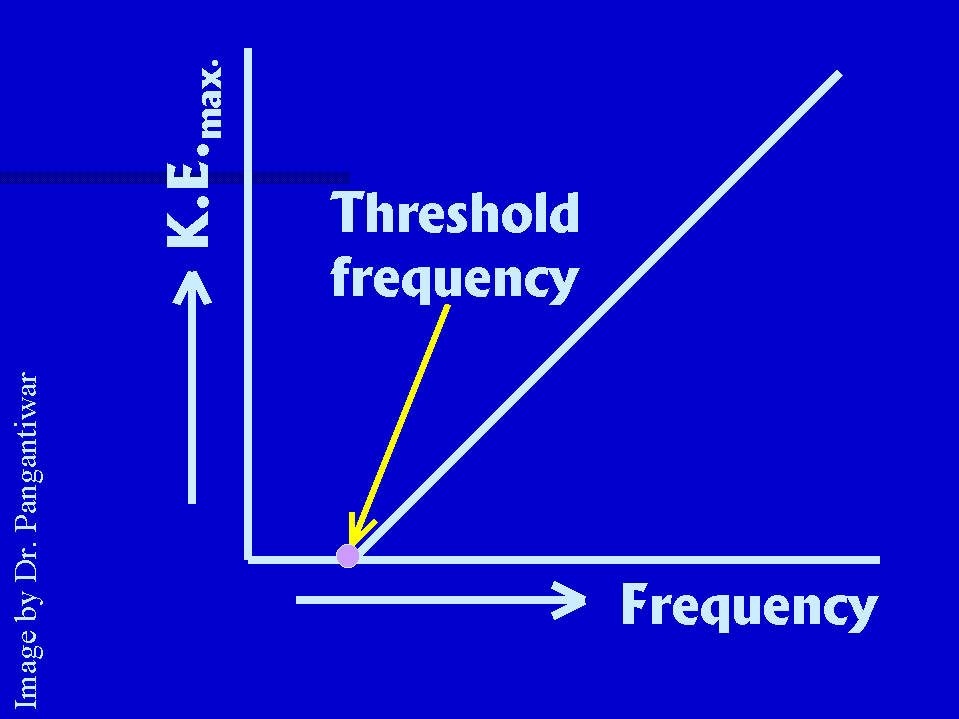
|
in a photoelectric effect experiment is shown in figure, which is a straight line.
The plot intersects the frequency
axis at threshold frequency, because the kinetic energy of photo-electron is equal to zero at
threshold frequency. Above threshold, the K.E. of photoelectrons is directly proportional to
the frequency of incident light, as the sketch is a straight line. According to quantum theory Energy of photon = Energy needed to liberate the |